Dental Spore Test Audits and Dental Spore Testing Regulations by State — Is Your Practice Inspection-ready?

Whether you’re an independent practice or an office affiliated with a larger healthcare system, sterilization and sterility assurance in healthcare facilities should be monitored using biological, mechanical, and chemical indicators.
Keeping track of your sterilization procedures protects your practice — and your patients. It’s mandatory for your inspections and has to be conducted at least weekly here in the US for your sterility assurance and risk management program.
A table-top sterilizer is defined in AAMI ST79’s “Comprehensive Guide To Sterility Assurance” as a compact steam sterilizer that has a chamber volume of less than or equal to 2 cubic feet and that generates its own steam when distilled or deionized water is added by the user.
It’s an essential piece of equipment in your medical or dental practice. So, what do you need for your weekly spore test when proof of testing is required?
What Needs To Be Included In The Logging Of Mechanical Quality Assurance?
Biological indicators, or spore tests, are the most accepted means of monitoring sterilization because they assess the sterilization process directly by killing known highly resistant microorganisms. However, because spore tests are only done weekly and the results are usually not obtained immediately.
There are three required methods of sterilization monitoring:
1. Mechanical. The gages read on the dashboard of the sterilizer
2. Chemical. Testing the parameters of steam penetration leading to sterilization but not confirming sterilization
3. Biological. Testing sterilization, the only means of confirming sterilization.
For record-keeping compliance, each test must be logged!
Items Specific To Sterilizer Quality Control
Via “Guide to Infection Prevention for Outpatient Settings: Minimum Expectations for Safe Care.”
— CDC, Version 2.3, Summary Guide
- Critical items (e.g., surgical instruments) are objects that enter sterile tissue or the vascular system and must be sterile prior to use.
- Cleaning must always be performed prior to sterilization and/or disinfection.
- Single-use devices (SUDs) are labeled by the manufacturer for a single-use and do not have reprocessing instructions. They may not be reprocessed for reuse except by entities that have complied with FDA regulatory requirements and have received FDA clearance to reprocess specific SUDs.
- Policies, procedures, and manufacturer reprocessing instructions for reusable medical devices used in the facility are available in the reprocessing area(s)
- HCP {Healthcare Personnel] responsible for reprocessing reusable medical devices receive hands-on training on proper selection and use of PPE.
- After cleaning, instruments are appropriately wrapped/packaged for sterilization E.G. package system selected is compatible with the sterilization process being performed, items are placed correctly into the basket, shelf, or cart of the sterilizer so as not to impede the penetration of the sterilant, hinged instruments are open, and instruments are disassembled if indicated by the manufacturer.
- Sterile packs are labeled with a load number that indicates the sterilizer used, cycle or load number, date of sterilization, and, if applicable, the expiration date.
- After sterilization, medical devices and instruments are stored so that sterility is not compromised.
The entire CDC checklist is available here.
Why not schedule sometime this week to perform a self-audit? Better yet, check out 1:1 risk management consulting with a Done Desk expert!
Our Features
Endless paperwork, manual tracking of deadlines, and scrambling to update protocols every time there's a regulatory tweak – it's enough to make anyone's head spin. Plus, it eats into precious time that could be better spent making your patients smile.
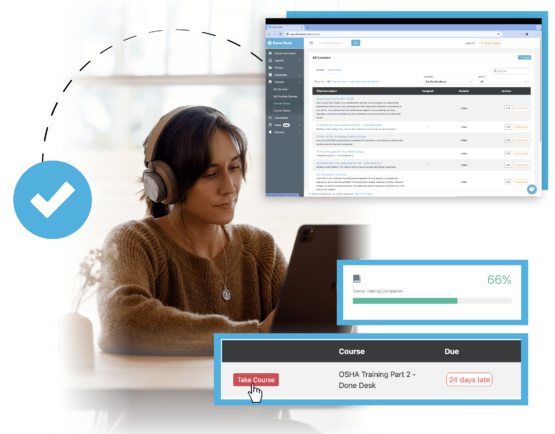
TRAINING & LMS
Integrated LMS allowing you to create or choose from a library of our pre-built training seamlessly.
Ensuring consistent training across multiple locations can be a challenge. With Done Desk's LMS, you can standardize training protocols, deliver engaging content, and track team progress.
Choose training topics from Done Desk's built-in training library - or create and upload your own training. Course Tracks allow you to build sequences of training — anything from a 30-day onboarding Track to a year-long CE Track. Get Training Done with Done Desk.
Your Office Documents
Centralized document hub - upload, organize, and sign documents.
With Done Desk Doc Automation, all your compliance documents – licenses, permits, policies – are neatly organized in one digital hub. Plus, with our SignableDocs feature — employees can sign required paperwork like W4s, I-9s, and more directly in-app!
Centralize document storage, enforce access controls, and maintain audit trails with ease. Rest assured that your organization is always prepared for inspections and audits, minimizing risk and safeguarding your reputation.

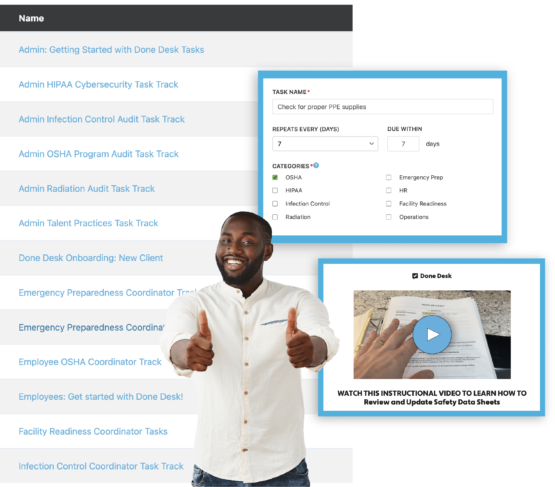
Tasks & Task Tracks
Create, assign, and monitor tasks across your organization.
Done Desk's pre-built library of Task Tracks get's you one step ahead — telling you what to do and how to do it! Then, keep track of things until it's time to do it again!
Create video Tasks and keep training employees continuously — or try out a repeating Task for things that need to get Done on a regular basis. If you can Task it — Done Desk helps get it Done.
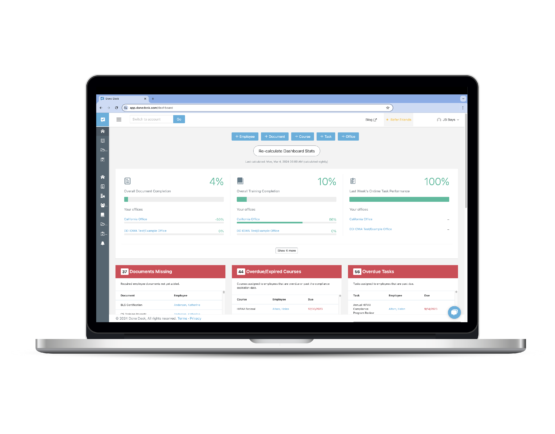
Done Desk for DSOs
Track adherence across multi-locations and quickly identify areas of non-compliance. Standardize your practices.
Done Desk keeps a detailed log of all your compliance activities office-by-office.
Done Desk grows with your organization, adapting to your evolving needs and driving continuous improvement. Experience the Done Desk difference and unlock the full potential of your organization today.
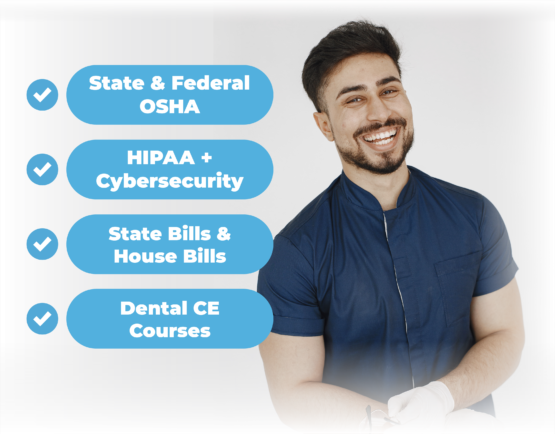
OSHA+HIPAA Built-In
Get the basics Done.
Get all your annual employee training Done with Done Desk! With our platform, you can ensure that your staff stays up-to-date and compliant with crucial regulations such as OSHA, HIPAA, and Continuing Education (CE) requirements.
Done Desk's LMS provides in-depth OSHA training modules covering topics such as infection control, hazardous materials handling, and emergency preparedness. With interactive content and real-world scenarios, our platform equips your team with the knowledge and skills they need to maintain a safe and healthy work environment.
Used & Loved by
Our Partners & Clients

“I use Done Desk to take the guesswork out of running Brush 365’s multiple offices! As the office manager, the platform keeps us efficient with employee files, timekeeping, onboarding, and the array of compliance regulations. Done Desk allows me to focus my time on the most important part of my role… leading my people!
The value of this platform simply cannot be underestimated.”

“I had literally been searching for something like Done Desk for over 5+ yrs. One of the most impressive things about Done Desk is the team. I greatly appreciate using a product where the founder is open & appreciative of feedback. How rare is that? And the communication goes two ways – with my feedback, they let me know the direction of growth they are focused on. In addition, the Done Desk team is exceptionally responsive, often within minutes of questions I have and this makes all the difference in the world.”







Hi! Want to keep up with Done Desk? We’ll make sure to only send interesting info, no crappy content or fluff. Just the good stuff — promise!
Get In Touch:
info@donedesk.com
(512) 222-3812
Follow Us!
9am - 5pm CST | Mon-Fri
Chat with us in the lower right!
Done Desk™
Software proudly designed and handmade in the USA.
Headquartered in San Antonio, Texas.
100% Staffed by real people in the USA.

Done Desk EDU is an approved PACE Program Provider for FAGD/MAGD credit and AGD Approved Courses
Approval does not imply acceptance by any regulatory authority or AGD endorsement.
Provider ID# 389654 | 3/1/2023 to 2/28/2026
Copyright Done Desk™ 2024

